Nepenthes
| Nepenthes | |
|---|---|
 |
|
| Upper pitcher of Nepenthes edwardsiana | |
| Scientific classification | |
| Kingdom: | Plantae |
| (unranked): | Angiosperms |
| (unranked): | Eudicots |
| (unranked): | Core eudicots |
| Order: | Caryophyllales |
| Family: | Nepenthaceae Dumort. |
| Genus: | Nepenthes L. |
| Species | |
|
See below or separate list. |
|
| Diversity | |
| >129 species[1] | |
| Synonyms | |
|
|
The Nepenthes (pronounced /nɨˈpɛnθiːz/, from Greek: ne 'not', penthos 'grief, sorrow'; named after the mythical drug Nepenthe), popularly known as tropical pitcher plants or monkey cups, are a genus of carnivorous plants in the monotypic family Nepenthaceae. The genus comprises roughly 120 species, numerous natural and many cultivated hybrids. They are mostly liana-forming plants of the Old World tropics, ranging from South China, Indonesia, Malaysia and the Philippines; westward to Madagascar (2 species) and the Seychelles (1); southward to Australia (3) and New Caledonia (1); and northward to India (1) and Sri Lanka (1). The greatest diversity occurs on Borneo and Sumatra with many endemic species. Many are plants of hot humid lowland areas, but the majority are tropical montane plants, receiving warm days but cool to cold humid nights year round. A few are considered tropical alpine with cool days and nights near freezing. The name monkey cups refers to the fact that monkeys have been observed drinking rainwater from these plants.
Contents |
Etymology
The name Nepenthes was first published in 1737 in Carolus Linnaeus's Hortus Cliffortianus.[2] It references a passage in Homer's Odyssey, in which the potion "Nepenthes pharmakon" is given to Helen by an Egyptian queen. "Nepenthe" literally means "without grief" (ne = not, penthos = grief) and, in Greek mythology, is a drug that quells all sorrows with forgetfulness. Linnaeus explained:
If this is not Helen's Nepenthes, it certainly will be for all botanists. What botanist would not be filled with admiration if, after a long journey, he should find this wonderful plant. In his astonishment past ills would be forgotten when beholding this admirable work of the Creator! [translated from Latin by Harry Veitch][3]
The plant Linnaeus described was Nepenthes distillatoria, a species from Sri Lanka.[4]
Nepenthes was formally published as a generic name in 1753 in Linnaeus's famous Species Plantarum, which established botanical nomenclature as it exists today. N. distillatoria is the type species of the genus.[5]

The name monkey cups was discussed in the May 1964 issue of National Geographic, in which Paul A. Zahl wrote:[6]
The carriers called them "monkey cups," a name I had heard elsewhere in reference to Nepenthes, but the implication that monkeys drink the pitcher fluid seemed farfetched. I later proved it true. In Sarawak I found an orangutan that had been raised as a pet and later freed. As I approached it gingerly in the forest, I offered it a half-full pitcher. To my surprise, the ape accepted it and, with the finesse of a lady at tea, executed a delicate bottoms-up.
Botanical history

The earliest known record of Nepenthes dates back to the 17th century. In 1658, French colonial governor Etienne de Flacourt published a description of a pitcher plant in his seminal work Histoire de la Grande Isle de Madagascar. It reads:[7]
It is a plant growing about 3 feet high which carries at the end of its leaves, which are 7 inches long, a hollow flower or fruit resembling a small vase, with its own lid, a wonderful sight. There are red ones and yellow ones, the yellow being the biggest. The inhabitants of this country are reluctant to pick the flowers, saying that if somebody does pick them in passing, it will not fail to rain that day. As to that, I and all the other Frenchmen did pick them, but it did not rain. After rain these flowers are full of water, each one containing a good half-glass. [translated from French in Pitcher-Plants of Borneo][4]
Flacourt called the plant Amramatico, after a local name. More than a century later, this species was formally described as N. madagascariensis.[8]
The second species to be described was N. distillatoria, the Sri Lankan endemic. In 1677, Bartholinus made brief mention of it under the name Miranda herba, Latin for "marvellous herb".[9] Three years later, Dutch merchant Jacob Breyne referred to this species as Bandura zingalensium, after a local name for the plant.[10] Bandura subsequently became the most commonly used name for the tropical pitcher plants, until Linnaeus coined Nepenthes in 1737.[4]
Nepenthes distillatoria was again described in 1683, this time by Swedish physician H. N. Grimm.[11] Grimm called it Planta mirabilis destillatoria or the "miraculous distilling plant", and was the first to clearly illustrate a tropical pitcher plant.[4] Three years later, in 1686, English naturalist John Ray quoted Grimm as saying:[12]
The root draws up moisture from the earth which with the help of the sun's rays rises up into the plant itself and then flows down through the stems and nerves of the leaves into the natural utensil to be stored there until used for human needs. [translated from Latin in Pitcher-Plants of Borneo][4]
One of the earliest illustrations of Nepenthes appears in Leonard Plukenet's Almagestum Botanicum of 1696.[13] The plant, called Utricaria vegetabilis zeylanensium, is undoubtedly N. distillatoria.[4]

It was around the same time that German botanist Georg Eberhard Rumphius discovered two new Nepenthes species in the Malay Archipelago. Rumphius illustrated the first one, now considered synonymous with N. mirabilis, and gave it the name Cantharifera, meaning "tankard-bearer". The second, referred to as Cantharifera alba, is thought to have been N. maxima. Rumphius described the plants in his most famous work, the six-volume Herbarium Amboinense, a catalogue of the flora of Ambon Island. However, it would not be published until many years after his death.[14]
After going blind in 1670, when the manuscript was only partially complete, Rumphius continued work on Herbarium Amboinensis with the help of clerks and artists. In 1687, with the project nearing completion, at least half of the illustrations were lost in a fire. Persevering, Rumphius and his helpers first completed the book in 1690. However, two years later, the ship carrying the manuscript to the Netherlands was attacked and sunk by the French, forcing them to start over from a copy that had fortunately been retained by Governor-General Johannes Camphuijs. The Herbarium Amboinensis finally arrived in the Netherlands in 1696. Even then, the first volume did not appear until 1741, thirty-nine years after Rumphius's death. By this time, Linnaeus's name Nepenthes had become established.[4]

Nepenthes distillatoria was again illustrated in Johannes Burmann's Thesaurus Zeylanicus of 1737. The drawing depicts the end of a flowering stem with pitchers. Burmann refers to the plant as Bandura zeylanica.[15]
The next mention of tropical pitcher plants was made in 1790, when Portuguese priest João de Loureiro described Phyllamphora mirabilis, or the "marvellous urn-shaped leaf", from Vietnam. Despite living in the country for around 35 years, it seems unlikely that Loureiro observed living plants of this species, as he states that the lid is a moving part, actively opening and closing. In his most celebrated work, Flora Cochinchinensis, he writes:[16]
[...] (the) leaf-tip ends in a long hanging tendril, twisted spirally in the middle, from which hangs a sort of vase, oblong, pot-bellied, with a smooth lip with a projecting margin and a lid affixed to one side, which of its own nature freely opens and closes in order to receive the dew and store it. A marvellous work of the Lord! [translated from French in Pitcher-Plants of Borneo][4]
Phyllamphora mirabilis was eventually transferred to the genus Nepenthes by George Claridge Druce in 1916.[17] As such, P. mirabilis is the basionym of this most cosmopolitan of tropical pitcher plant species.[18]
Loureiro's description of a moving lid was repeated by Jean Louis Marie Poiret in 1797. Poiret described two of the four Nepenthes species known at the time: N. madagascariensis and N. distillatoria. He gave the former its current name and called the latter Nepente de l'Inde, or simply "Nepenthes of India", although this species is absent from the mainland. In Jean-Baptiste Lamarck's Encyclopédie Méthodique Botanique, he included the following account:[8]
This urn is hollow, as I have just said, usually full of soft, clear water, and then closed. It opens during the day and more than half the liquid disappears, but this loss is repaired during the night, and the next day the urn is full again and closed by its lid. This is its sustenance, and enough for more than one day because it is always about half-full at the approach of night. [translated from French in Pitcher-Plants of Borneo][4]

With the discovery of new species and Sir Joseph Banks' original introduction of specimens to Europe in 1789, interest in Nepenthes grew throughout the 19th century, culminating in what has been called the "Golden Age of Nepenthes" in the 1880s.[4][19] However, the popularity of the plants dwindled in the early 20th century, before all but disappearing by World War II. This is evidenced by the fact that no new species were described between 1940 and 1966. The revival of global interest in the cultivation and study of Nepenthes is credited to Japanese botanist Shigeo Kurata, whose work in the 1960s and 1970s did much to bring attention to these plants.[20]
Description
Nepenthes species usually consist of a shallow root system and a prostrate or climbing stem, often several metres long and up to 15 m (49 ft) or more, and usually 1 cm (0.4 in) or less in diameter, although this may be thicker in a few species (e.g. N. rafflesiana). From the stems arise alternate sword-shaped leaves with entire leaf margins. An extension of the midrib (tendril), which in some species aid in climbing, protrudes from the tip of the leaf; at the end of the tendril the pitcher forms. The pitcher starts as a small bud and gradually expands to form a globe- or tube-shaped trap.[19]
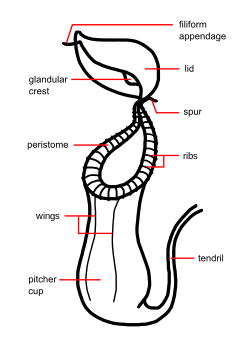
The trap contains a fluid of the plant's own production, which may be watery or syrupy and is used to drown the prey. Research has shown that this fluid contains viscoelastic biopolymers that may be crucial to the retention of insects within the traps of many species. The trapping efficiency of this fluid remains high, even when significantly diluted by water, as inevitably happens in wet conditions.[21]
The lower part of the trap contains glands which absorb nutrients from captured prey. Along the upper inside part of the trap is a slick waxy coating which makes the escape of its prey nearly impossible. Surrounding the entrance to the trap is a structure called the peristome (the "lip") which is slippery and often quite colorful, attracting prey but offering an unsure footing. Above the peristome is a lid (the operculum): in many species this keeps rain from diluting the fluid within the pitcher, the underside of which may contain nectar glands which attract prey.[19]
Nepenthes usually produce two types of pitchers, known as leaf dimorphism. Appearing near the base of the plant are the large lower traps, which typically sit on the ground. The upper or aerial pitchers are usually smaller, differently-coloured, and possess different features than the lower pitchers. These upper pitchers usually form as the plant reaches maturity and the plant grows taller. To keep the plant steady, the upper pitchers often form a loop in the tendril, allowing it to wrap around nearby support. In some species (e.g. N. rafflesiana) different prey may be attracted by the two types of pitchers. This varied morphology also often makes identification of species difficult.[19]
Prey usually consists of insects, but the largest species (e.g. N. rajah and N. rafflesiana) may occasionally catch small vertebrates, such as rats and lizards.[22][23] Flowers occur in racemes or more rarely in panicles with male and female flowers on separate plants. Seed is produced in a four-sided capsule which may contain 50–500 wind-distributed seeds, consisting of a central embryo and two wings, one on either side.
Distribution and habitat

The genus Nepenthes is mostly found within the Malay Archipelago, with the greatest biodiversity found on Borneo and Sumatra, especially in the Borneo montane rain forests. The full range of the genus includes Madagascar (N. madagascariensis and N. masoalensis), the Seychelles (N. pervillei), Sri Lanka (N. distillatoria), and India (N. khasiana) in the west to Australia (N. mirabilis, N. rowanae, and N. tenax) and New Caledonia (N. vieillardii) in the southeast. Most species are restricted to very small ranges, including some that are only found on individual mountains. These limited distributions and the inaccessibility of the region often means that some species go decades without being rediscovered in the wild (e.g. N. deaniana, which was rediscovered 100 years after its initial discovery). Approximately ten species have population distributions larger than a single island or group of smaller islands. Nepenthes mirabilis has the distinction of being the most widely distributed species in the genus, ranging from Indochina and throughout the Malay Archipelago to Australia.[19][24][25]
Because of the nature of the habitats which Nepenthes species occupy, they are often graded as either lowland or highland species, depending on their altitude above sea level, with 1,200 m (3,937 ft) the rough delineation between lowland and highland. Species that grow at lower altitudes require continuously warm climates with little difference between day and night temperatures, whereas highland species thrive when they receive warm days and much cooler nights. Nepenthes lamii is the species that holds the record of growing at a higher altitude than any other in the genus, up to 3,520 m (11,549 ft).[19][25]
Most Nepenthes species grow in environments that provide high humidity and precipitation and moderate to high light levels. A few species, including N. ampullaria, prefer the dense, shaded forests, but most other species thrive on the margins of tree/shrub communities or clearings. Some species (e.g. N. mirabilis) have been found growing in clear-cut forest areas, roadsides, and disturbed fields. Other species have adapted to growing in savanna-like grass communities. The soils that Nepenthes grow in are usually acidic and low in nutrients, being composed of peat, white sand, sandstone, or volcanic soils. There are, however, exceptions to these generalities, including species that thrive in soils that have high heavy metal content (e.g. N. rajah), on sandy beaches in the sea spray zone (e.g. N. albomarginata). Other species grow on inselbergs and as lithophytes, while others, such as N. inermis, can grow as epiphytes with no soil contact.[19]
Ecological relationships

The most obvious interaction between Nepenthes species and its environment, including other organisms, is that of predator and prey. Nepenthes species certainly attract and kill their prey, albeit passively, through active production of attractive colours, sugary nectar, and even sweet scents. From this relationship, the plants primarily gain nitrogen and phosphorus to supplement their nutrient requirements for growth, given that soil nutrients are typically lacking. The most frequent prey is an abundant and diverse group of arthropods, with ants and other insects topping the menu. Other arthropods that are found frequently include spiders, scorpions, and centipedes, while snails and frogs are more unusual but not unheard of. The most uncommon prey for Nepenthes species includes rats found in N. rajah. The composition of prey captured depends on many factors, including location, but can incorporate hundreds of individual insects and many different species.[19]
Symbioses
Some Nepenthes have developed unique, symbiotic relationships with prey, such as N. albomarginata, which almost exclusively traps termites yet produces nearly no nectar. Nepenthes albomarginata gains its name from the ring of white trichomes that are directly beneath the peristome. These trichomes—or "hairs"—are palatable to termites and will attract them to the pitcher. In the course of collecting the edible trichomes, many termites will fall into the pitcher, which is an acceptable loss to the termite colony for the food provided by the plant.[26]

Another species, N. bicalcarata, provides space in its hollow tendrils of its upper pitchers for the carpenter ant Camponotus schmitzi to build nests. The ants take larger prey from the pitchers, which may benefit N. bicalcarata by reducing the amount of putrefaction of collected organic matter that could harm the natural community of infaunal species that aid the plant's digestion.[18]
Nepenthes lowii has also formed a dependent relationship, but this time it is with vertebrates instead of insects. The pitchers of N. lowii provide a sugary exudate reward on the reflexed pitcher lid (operculum) and a perch for tree shrew species, which have been found eating the exudate and defecating into the pitcher. A 2009 study, which coined the term "tree shrew lavatories", determined that anywhere between 57 and 100% of the plant's foliar nitrogen uptake comes from the faeces of tree shrews.[28] Another study published the following year showed that the shape and size of the pitcher orifice of N. lowii exactly match the dimensions of a typical tree shrew (Tupaia montana).[29][30] A similar adaptation was found in N. macrophylla and N. rajah, and is also likely to be present in N. ephippiata.[30]
Infauna
Organisms that spend at least part of their lives within the pitchers of Nepenthes species are often called Nepenthes infauna. The most common infaunal species, often representing the top trophic level of the infaunal ecosystem, are many species of mosquito larvae. Other infaunal species include fly and midge larvae, spiders, mites, ants, and even a species of crab (Geosesarma malayanum). Many of these species specialise to one pitcher plant species and are found nowhere else. These specialists are called nepenthebionts. Others that are often associated but are not dependent on Nepenthes species are called nepenthophiles. Nepenthexenes, on the other hand, are rarely found in the pitchers, but will often appear when putrefaction approaches a certain threshold, attracting fly larvae that would normally not be found in the pitcher infaunal community. The complex ecological relationship between pitcher plant and infauna is not yet fully understood, but it has been suggested that the relationship is mutualistic: the infauna is given shelter, food, or protection and the plant that harbours the infauna receives expedited breakdown of captured prey, increasing the rate of digestion and keeping harmful bacterial populations repressed.[18][31][32]
Cultivation

Nepenthes may be cultivated in greenhouses. Easier species include N. alata, N. ventricosa, N. khasiana, and N. sanguinea. These four species are highlanders (N. alata has both lowland and highland forms), some easy lowlander species are N. rafflesiana, N. bicalcarata, N. mirabilis, and N. hirsuta.
Highland forms are those species that grow in habitats that are generally higher up in elevation, and thus exposed to cooler evening temperatures. Lowland forms are those species that grow nearer to sea level. Both forms respond best to rainwater (but tap water works as long as you flush it out with rainwater every month or if you have soft water), bright light (not full sun), a well drained medium, good air circulation and relatively high humidity, although easier species such as N. alata can adapt to lower humidity environments. Highland species must have night-time cooling to thrive in the long-term. Chemical fertilisers are best used at low strength. Occasional feeding with frozen (thawed before use) crickets may be beneficial. Terrarium culture of smaller plants like N. bellii, N. × trichocarpa and N. ampullaria is possible, but most plants will get too large over time.
Plants can be propagated by seed, cuttings, and tissue culture. Seeds are usually sown on damp chopped Sphagnum moss, or on sterile plant tissue culture media once they have been properly disinfected. The seeds generally become nonviable soon after harvesting, so seed are not usually the preferred method of propagation. A 1:1 mixture of orchid medium with moss or perlite has been used for germination and culture. Seed may take two months to germinate, and two years or more to yield mature plants. Cuttings may be rooted in damp Sphagnum moss in a plastic bag or tank with high humidity and moderate light. They can begin to root in 1–2 months and start to form pitchers in about six months. Tissue culture is now used commercially and helps reduce collection of wild plants, as well as making many rare species available to hobbyists at reasonable prices. Nepenthes are considered threatened or endangered plants and are listed in CITES appendices 1 & 2.
Species
More than 100 species of Nepenthes are currently recognised as valid. This number is fast increasing, with several new species being described each year.[20]
Hybrids and cultivars



There are many hybrid Nepenthes and numerous named cultivars. The following are named natural hybrids:
- N. × alisaputrana (N. burbidgeae × N. rajah)
- N. × bauensis (N. gracilis × N. northiana)
- N. × cantleyi (N. bicalcarata × N. gracilis)
- N. × cincta (N. albomarginata × N. northiana)
- N. × ferrugineomarginata (N. albomarginata × N. reinwardtiana)
- N. × harryana (N. edwardsiana × N. villosa)
- N. × hookeriana (N. ampullaria × N. rafflesiana)
- N. × kinabaluensis (N. rajah × N. villosa)
- N. × kuchingensis (N. ampullaria × N. mirabilis)
- N. × merrilliata (N. alata × N. merrilliana)
- N. × mirabilata (N. alata × N. mirabilis)
- N. × pangulubauensis (N. gymnamphora × N. mikei)
- N. × pyriformis (N. inermis × N. talangensis)
- N. × sarawakiensis (N. muluensis × N. tentaculata)
- N. × sharifah-hapsahii (N. gracilis × N. mirabilis)
- N. × trichocarpa (N. ampullaria × N. gracilis)
- N. × truncalata (N. alata × N. truncata)
- N. × trusmadiensis (N. lowii × N. macrophylla)
- N. × tsangoya ((N. alata × N. merrilliana) × N. mirabilis)
- N. × ventrata (N. alata × N. ventricosa)
Some of the more well known artificially produced hybrids include:
- N. 'Coccinea' ((N. rafflesiana × N. ampullaria) × N. mirabilis)
- N. 'Emmarene' (N. khasiana × N. ventricosa)
- N. 'Gentle' (N. fusca × N. maxima)
- N. 'Judith Finn' (N. veitchii × N. spathulata)
- N. 'Miranda' ((N. maxima × N. northiana) × N. maxima)
- N. 'Mixta' (N. northiana × N. maxima)
See also
- Nepenthes classification
- Nepenthes infauna
References
- ↑ McPherson, S.R. 2010. Carnivorous Plants and their Habitats. Redfern Natural History Productions Ltd., Poole.
- ↑ Linnaeus, C. 1737. Nepenthes. Hortus Cliffortianus. Amsterdam.
- ↑ Veitch, H.J. 1897. Nepenthes. Journal of the Royal Horticultural Society 21(2): 226–262.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Phillipps, A. & A. Lamb 1996. Pitcher-Plants of Borneo. Natural History Publications (Borneo), Kota Kinabalu.
- ↑ Linnaeus, C. 1753. Nepenthes. Species Plantarum 2: 955.
- ↑ Zahl, P.A. 1964. Malaysia's Giant Flowers and Insect-trapping Plants. National Geographic 125(5): 680–701.
- ↑ de Flacourt, E. 1658. Histoire de la Grande Isle de Madagascar.
- ↑ 8.0 8.1 Poiret, J.L.M. 1797. Népente. In: J.B. Lamarck Encyclopédie Méthodique Botanique Vol. 4.
- ↑ Bartholinus 1677. Miranda herba. Acta Medica et Philosophica Hafniensia 3: 38.
- ↑ Breyne, J. 1680. Bandura zingalensium etc. Prodromus Fasciculi Rariorum Plantarum 1: 18.
- ↑ Grimm, H.N. 1683. Planta mirabilis destillatoria. In: Miscellanea curiosa sive Ephemeridum. Med. Phys. Germ. Acad. Nat. Cur. Decuriae 2, ann. prim. p. 363, f. 27.
- ↑ Ray, J. 1686. Bandura cingalensium etc. Historia Plantarum 1: 721–722.
- ↑ Plukenet, L. 1696. Utricaria vegetabilis zeylanensium. In: Almagestum Botanicum.
- ↑ Rumphius, G.E. 1741–1750. Cantharifera. In: Herbarium Amboinense 5, lib. 7, cap. 61, p. 121, t. 59, t. 2.
- ↑ Burmann, J. 1737. Thesaurus Zeylanicus. Amsterdam.
- ↑ de Loureiro, J. 1790. Flora Cochinchinensis 2: 606–607.
- ↑ Druce, G. 1916. Nepenthes mirabilis. In: Botanical Exchange Club of the British Isles Report 4: 637.
- ↑ 18.0 18.1 18.2 Clarke, C.M. 1997. Nepenthes of Borneo. Natural History Publications (Borneo), Kota Kinabalu.
- ↑ 19.0 19.1 19.2 19.3 19.4 19.5 19.6 19.7 Barthlott, W., Porembski, S., Seine, R., and Theisen, I. 2007. The Curious World of Carnivorous Plants. Portland, Oregon: Timber Press.
- ↑ 20.0 20.1 Clarke, C.M. & C.C. Lee 2004. Pitcher Plants of Sarawak. Natural History Publications (Borneo), Kota Kinabalu.
- ↑ Gaume, L. & Y. Forterre 2007. A Viscoelastic Deadly Fluid in Carnivorous Pitcher Plants. PLoS ONE 2(11): e1185. doi:10.1371/journal.pone.0001185
- ↑ Phillipps, A. 1988. A Second Record of Rats as Prey in Nepenthes rajah. Carnivorous Plant Newsletter 17(2): 55.
- ↑ Moran, J.A. 1991. The role and mechanism of Nepenthes rafflesiana pitchers as insect traps in Brunei. Ph.D. thesis, University of Aberdeen, Aberdeen, Scotland.
- ↑ McPherson, S.R. 2009. Pitcher Plants of the Old World. Redfern Natural History Productions Ltd., Poole.
- ↑ 25.0 25.1 Jebb, M., and Cheek, M. 1997. A skeletal revision of Nepenthes (Nepenthaceae). Blumea, 42: 1-106.
- ↑ Moran, J.A., Merbach, M.A., Livingston, N.J., Clarke, C.M., and Booth, W.E. 2001. Termite prey specialization in the pitcher plant Nepenthes albomarginata—evidence from stable isotope analysis. Annals of Botany, 88: 307-311.
- ↑ Robinson, A.S., A.S. Fleischmann, S.R. McPherson, V.B. Heinrich, E.P. Gironella & C.Q. Peña 2009. A spectacular new species of Nepenthes L. (Nepenthaceae) pitcher plant from central Palawan, Philippines. Botanical Journal of the Linnean Society 159(2): 195–202. doi:10.1111/j.1095-8339.2008.00942.x
- ↑ Clarke, C.M., U. Bauer, C.C. Lee, A.A. Tuen, K. Rembold & J.A. Moran 2009. Tree shrew lavatories: a novel nitrogen sequestration strategy in a tropical pitcher plant.PDF Biology Letters 5(5): 632–635. doi:10.1098/rsbl.2009.0311
- ↑ Chin, L., J.A. Moran & C. Clarke 2010. Trap geometry in three giant montane pitcher plant species from Borneo is a function of tree shrew body size. New Phytologist 186 (2): 461–470. doi:10.1111/j.1469-8137.2009.03166.x
- ↑ 30.0 30.1 Walker, M. 2010. Giant meat-eating plants prefer to eat tree shrew poo. BBC Earth News, March 10, 2010.
- ↑ Mogi, M., and Yong, H.S. 1992. Aquatic arthropod communities in Nepenthes pitchers: the role of niche differentiation, aggregation, predation and competition in community organization. Oecologia, 90(2): 172-184. doi:10.1007/BF00317174
- ↑ Beaver, R.A. 1979. Fauna and food webs of pitcher plants in West Malaysia. Malayan Nature Journal, 33: 1-10.
- Danser's Monograph on Nepenthes (covers species from Malaysia, Indonesia and New Guinea, but not elsewhere)
- Nepenthaceae in: Watson, L., and M. J. Dallwitz (1992 onwards). The Families of Flowering Plants. Descriptions, Illustrations, Identification, Information Retrieval.
Further reading
- Amagase, S., S. Nakayama & A. Tsugita 1969. Acid protease in Nepenthes. II. Study on the specificity of nepenthesin. The Journal of Biochemistry 66(4): 431–439.
- Athauda, S.B.P., K. Matsumoto, S. Rajapakshe, M. Kuribayashi, M. Kojima, N. Kubomura-Yoshida, A. Iwamatsu, C. Shibata, H. Inoue & K. Takahashi 2004. Enzymatic and structural characterization of nepenthesin, a unique member of a novel subfamily of aspartic proteinases.PDF (1.32 MB) (manuscript BJ20031575) Biochemical Journal 381(1): 295–306. doi:10.1042/BJ20031575
- Bauer, U., Bohn, H.F. & Federle, W. 2008. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar.PDF Proceedings of the Royal Society B 275(1632): 259-265.
- Beaver, R.A. 1979. Biological studies of the fauna of pitcher plants Nepenthes in west Malaysia. Annales de la Société Entomologique de France 15: 3–17.
- Beaver, R.A. 1979. Fauna and foodwebs of pitcher plants in west Malaysia. Malayan Nature Journal 33: 1–10.
- Beaver, R.A. 1983. The communities living in Nepenthes pitcher plants: fauna and food webs. In: J.H. Frank & L.P. Lounibos (eds.) Phytotelmata: Plants as Hosts for Aquatic Insect Communities. Plexus Publishing, New Jersey. pp. 129–159.
- Beaver, R.A. 1985. Geographical variation in food web structure in Nepenthes pitcher plants. Ecological Entomology 10: 241–248.
- Beekman, E.M. 2004. A Note on the Priority of Rumphius' Observation of Decapod Crustacea Living In Nepenthes. Crustaceana 77(8): 1019–1021. doi:10.1163/1568540042781748
- Bohn, H.F. & W. Federle 2004. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface.PDF (547 KB) Proceedings of the National Academy of Sciences 101(39): 14138–14143. doi:10.1073/pnas.0405885101
- Carlquist, S. 1981. Wood Anatomy of Nepenthaceae. Bulletin of the Torrey Botanical Club 108(3): 324–330. doi:10.2307/2484711
- Chia, T.F., H.H. Aung, A.N. Osipov, N.K. Goh & L.S. Chia 2004. Carnivorous pitcher plant uses free radicals in the digestion of prey. Redox Report 9(5): 255–261. doi:10.1179/135100004225006029
- Frazier, C.K. 2000. The Enduring Controversies Concerning the Process of Protein Digestion in Nepenthes. Carnivorous Plant Newsletter 29(2): 56–61.
- Meimberg, H., A. Wistuba, P. Dittrich & G. Heubl 2001. Molecular Phylogeny of Nepenthaceae Based on Cladistic Analysis of Plastid trnK Intron Sequence Data. Plant Biology (Stuttgart, Germany) 3: 164–175. doi:10.1055/s-2001-12897
- Mogi, M. & H.S. Yong 1992. Aquatic arthropod communities in Nepenthes pitchers: the role of niche differentiation, aggregation, predation and competition in community organization. Oecologia 90: 172–184.
- Mokkamul, P., A. Chaveerach, R. Sudmoon & T. Tanee 2007. Species Identification and Sex Determination of the Genus Nepenthes (Nepenthaceae).PDF (702 KB) Pakistan Journal of Biological Sciences 10(4): 561–567. doi:10.3923/pjbs.2007.561.567
- Moran, J.A., W.E. Booth & J.K. Charles 1999. Aspects of Pitcher Morphology and Spectral Characteristics of Six Bornean Nepenthes Pitcher Plant Species: Implications for Prey Capture.PDF (187 KB) Annals of Botany 83: 521–528.
- Osunkoya, O.O., S.D. Daud, B. Di-Giusto, F.L. Wimmer & T.M. Holige 2007. Construction Costs and Physico-chemical Properties of the Assimilatory Organs of Nepenthes Species in Northern Borneo. Annals of Botany 99(5): 895–906. doi:10.1093/aob/mcm023
- Pavlovič, A., E. Masarovičová & J. Hudák 2007. Carnivorous Syndrome in Asian Pitcher Plants of the Genus Nepenthes. Annals of Botany 100(3): 527–536. doi:10.1093/aob/mcm145
- Riedel, M., A. Eichner, H. Meimberg & R. Jetter 2007. Chemical composition of epicuticular wax crystals on the slippery zone in pitchers of five Nepenthes species and hybrids. Planta 225(6): 1517–1534. doi:10.1007/s00425-006-0437-3
- Schulze, W., W.B. Frommer & J.M. Ward 1999. Transporters for ammonium, amino acids and peptides are expressed in pitchers of the carnivorous plant Nepenthes.PDF The Plant Journal 17(6): 637–646. doi:10.1046/j.1365-313x.1999.00414.x
- Vines, S.H. 1876. On the Digestive Ferment of Nepenthes. Journal of Anatomy and Physiology 11(1): 124–127.
External links
- Nepenthes - the Monkey Cups from the Botanical Society of America
- Photographs of Nepenthes in their natural habitats by Joachim Nerz
- Nepenthes photographs at the Carnivorous Plant Photo Finder
- A video about Nepenthes from The Private Life of Plants
- The Carnivorous Plant FAQ: Nepenthes by Barry Rice
|
||||||||||||||